Bradykinin
Modifications of the terminal
parts of the peptide type ligands resulted significant increases and decreases
in activity (e.g. bradykinin derivatives) Kõhidai et al.
(2002),
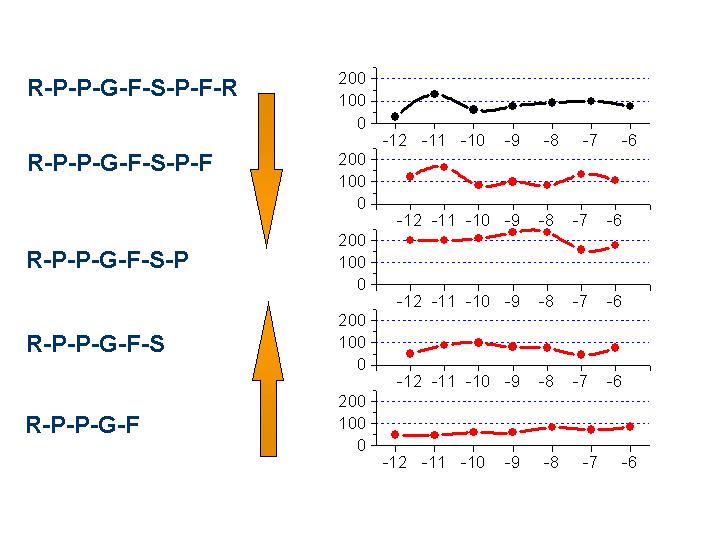
Chemotactic effect of
bradykinin (RPPGFSPFR) and derivatives. Truncation of the ligand results loss in
chemoattractant moiety, however expression of Phe on the N-terminus overridges
the chemotactic ability of the native (BK1-9) ligand.
Lecture
►
Endothelin
The increased
number of aromatic amino acid residues in the interacting regions of the
molecules were responsible for significant loss in chemotactic ability (e.g
endothelins) (Kõhidai
et al. 2001).
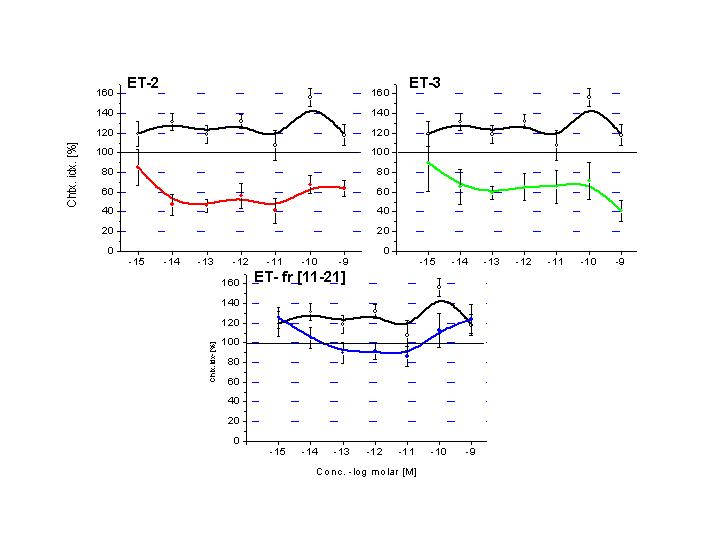
Chemotaxis induced with
endothelins. Black line represents ET-1 - the only wide range chemoattractant
endothelin derivative. Presence of aromatic amino acids in the loop-region of
the molecule (ET-2 and ET-3) results chemorepellent ligands, while the linear
fragment (ETfr11-21) still possess sligh chemoattractant moiety.
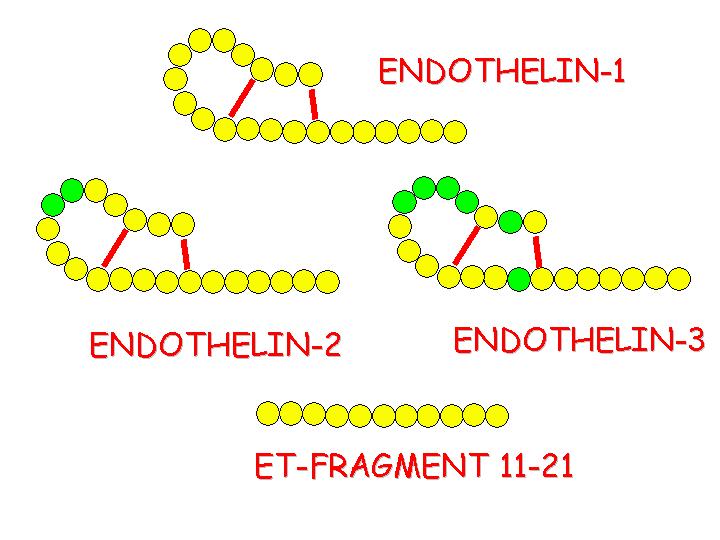
Structure of endothelins.
Circles filled green represent amino acids with aromatic sidechains.


